


With compounding pharmacy regulations under scrutiny, compliance has never been more important. Maintain control and consistency in your compounding procedures with solutions from Charles River. Offering unmatched regulatory expertise and a portfolio of FDA-licensed products and GMP-compliant services, Charles River is the trusted partner who can help you align your operations with current regulatory standards.
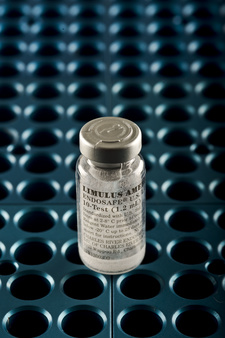
Required by regulatory bodies for compounding pharmacies, the Bacterial Endotoxins Test (BET) detects unsafe levels of microbial cell wall debris, from live or dead Gram-negative bacteria, that cause fever or septic shock. Unfortunately, traditional BET methods can be costly, requiring skilled analysts and manipulation of cumbersome reagents. The Endosafe® nexgen-PTS™ is a more efficient way to conduct this critical testing. The convenience of the handheld spectrophotometer simplifies endotoxin testing to deliver real-time, point-of-sample quantitative endotoxin results in just 15 minutes. All necessary LAL reagents are condensed into a single disposable cartridge for easy operation and assurance that your products are free of endotoxin within limits set by the United States Pharmacopeia (USP).
Our Endosafe® nexgen-PTS™ endotoxin testing systems provide faster results for improved process efficiency and ease-of-use over traditional methods, while offering additional benefits of portability, improved accuracy, and reproducibility. The LAL cartridges used with the system are licensed by the FDA for in-process and final product release testing of parenterals, such as compounded sterile products (CSPs). The nexgen-PTS™ is fully compatible with USP chapters <85> Bacterial Endotoxins Test and <797> Sterile Compounding, and is the optimum solution for bacterial endotoxins testing of compounded drugs due to its simplicity and speed.
Confident microbial and particle measurement is critical for your environmental monitoring program to ultimately confirm the security of your compounding area. Accurately identifying an organism to the species, and many times, to the strain level, facilitates tracking of the potential origin of the contamination and prevents delays in product release and completion of investigations.
Charles River offers comprehensive contract microbial testing services from our FDA-registered, cGMP-compliant laboratories. We have supported QC testing for over 1,000 global facilities with our cost-effective microbial identification and strain typing services using our proprietary Accugenix® database, which profiles more relevant microorganisms than any other reference library in the industry. Offering a 98% accurate identification rate and 99% on-time delivery, we have tested and identified more microorganisms than any other company or service laboratory in the industry.
Whether faced with critical timelines or stringent regulations, you can rely on Charles River to ensure the safe manufacture and timely release of your product.
 RXinsider | The preceding content is a booth exhibit in
RXinsider's Virtual Pharmacy Trade Show.
RXinsider | The preceding content is a booth exhibit in
RXinsider's Virtual Pharmacy Trade Show.
Share booth #16110 with others.